Microscopic Influencers: How Notch1 Inspires to“Stick” Together
Similar to how influencers use their platforms to convince their followers to partake in something, our cells use specific proteins like platforms to communicate different messages depending on what the body needs at that time. Adhesion proteins help cells to hold tightly to one another and make a barrier, for example in the intestines and in the brain. However, we are now realizing that these adhesions do more than just hold tightly: they also act as influencers, sending messages to the cells on both sides of the interaction to perform different tasks. One particular type of protein is called Notch. Its name comes from the original experiments in fruit flies which noticed that flies missing this protein had a “notch” on their wings. At least this name makes more sense than the protein called “Sonic Hedgehog” (real protein!). As it turns out, the Notch family is an integral regulator of developmental patterning in cells. Composed of a large extracellular polypeptide domain (ECD), a transmembrane domain (TMD), and an intracellular domain (ICD), these receptors are a key influencer which determine the fates of adjunct cells. If we think of a lock and key, the Notch receptors would be the lock and the ligands the key. Interactions between these two are crucial to unlocking cell signaling events. Ligands interact with the outside of Notch receptors and unleash a cascade of events on the side of the Notch receptor inside the cell. Inside the cell this signal is able to trigger the changing of gene expression, or how our genetic code is written and executed in the form of determining cell function. However, the regulation of these pathways remains largely unknown, especially in key pathways such as Notch.
Past research has shown how the Notch1 receptor is activated in vascular cells, particularly in how the TMD acts as a point of protein-protein interactions to promote the building of vascular cell barriers in a process known as “Notch1 cortical signaling”. The “cortical” here refers to the concentration of structural proteins that exists on the inside of the cell membrane. However, knowing how cortical signaling works in vascular cells doesn’t necessarily dictate how these pathways are regulated in epithelial cells (such as the ones in your skin, or intestines), as there are substantial structural and signaling differences between both cells. The question remains: How does Notch1 cortical signaling influence epithelial architecture and cell-cell adhesion?
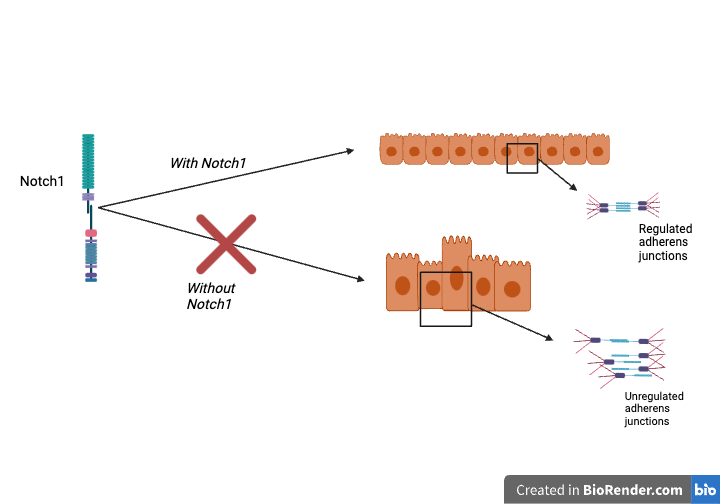
The lab of Matthew Kutys seeked to explore this question, and published their findings in the Journal of Cell Biology. The lab found that Notch1 influenced the determination of cell shape independently from ICD transcriptional signaling using the mammary duct epithelium as a model type of cell. The researchers used CRISPR-Cas9 gene editing as a way to cut and paste certain parts of the genome that they wish to edit. It essentially “cuts'' DNA at the point of interest and opens it up to insert any new material. In this experiment, the team used CRISPR to cut the ICD and shorten it. An RNA strand (SCR) was used as a control. During the experiment, normal “control” cells were able to form a protective monolayer over duct cells lined by columns of growth arrested epithelial cells. In contrast, the cells lacking Notch “NotchKO'' developed bent duct architectures with out-of-control tissue overgrowth which created large variances in duct diameter. The center of the cell ducts (the lumen) were also blocked compared to the SCR cells, leading to a decreased lumen diameter and thus increased cell packing. There was also a failure of monolayer development from the NotchKO cells. In investigating ICDKO ducts, White et. al found that the duct architecture bore resemblance to control ducts. This vast difference in NotchKO cells signifies a specific function for transcription-independent Notch1 cortical signaling in regulating the structure and assembly of a 3D engineered ductal epithelium. The authors concluded that dysregulated architecture and proliferation could be associated with a loss of Notch1 cortical signaling.
The authors also looked into certain pathways which led into increased epithelial cell reproduction. By comparing skin growth factor (EGF, epidermal growth factor, hormone) activity in cells, Notch1 was found to negatively regulate EGF hormone sensitivity and the hormone signal cascade in mammal cells. The data from White et al’s rigorous work supports increased EGFR cascade activity leads to an odd epithelial cell reproduction cycle observed upon the loss of Notch1 cortical signaling, but that the EGFR activity isn’t responsible for defects in the mammary epithelial cell architecture and cell organization.
One major question that arose with these experiments is how does the TMD affect cell morphology? To answer this question, Kutys’ lab looked into FAM83H, a band of proteins found in the cell. FAM83H is a series of genes known to be linked to cancer progression and in cell shape development. The team wanted to discover if FAM83H could be the determining difference in those lacking Notch1 cortical signaling, and thus engineered a 3D duct without FAM83H to see if it was missing any key features commonly associated with Notch1 signaling. They found that FAM83H has no effect on Notch1 signaling, and acts downstream (or later on) in the Notch1 pathway. The data suggests that FAM83H is a new Notch1 signaling effector, one that acts as a stabilizer of epithelial adherens junctions and cortical muscle organization.
The summation of these findings yields the fact that Notch1 controls epithelial cell architecture by regulating cell-linkage functions (adherens junctions) independent of ICD transcription (Figure). One strength of this study is that they dissected the genotypic and phenotypic effects of Notch, assigning specific functions to different parts of the molecule. The intracellular part of Notch is well known to control proliferation and cell fate. For future studies, understanding how Notch1 cortical and transcriptional signaling cooperate in cell differentiation and fate and adherens junctions can tell more about tissue development and homeostasis. This study also tells us more about how cell-cell adhesion can be regulated. With Notch1 signaling influencing changes in adherens junctions, the possibilities of new cell shape patterns open up through manipulating the cell communication in these adherens junctions. The paper raises hope for treating complications relating to skin barrier defects with faulty Notch1 tumor suppression, which would give hope to those seeking treatment for various skin cancer and tissue jamming defects during cell development.
Extra Q&A with Dr. Kutys:
In your study you created an artificial model of a mammalian epithelial cell duct and you previously showed a specific role of cortical Notch i endothelial cells. I was curious whether this cortical Notch/Fam83H pathway is active in any other type of cells?
You’re exactly right in highlighting the important functional and architectural differences between endothelial and epithelial cells. FAM83H and Notch1 appears to be a pathway unique to epithelial cells and is in fact active in other epithelial cell types. We’ve been recently focusing on stratified squamous epithelial cells models, including the epidermis and oral mucosa, where we see phenotypes upon loss of FAM83H. Importantly these models are now allowing us to parse the cortical and transcriptional signaling arms of Notch during developmental morphogenesis.
Secondly, I understand that Notch is typically activated through interactions with a ligand; where is the ligand expressed?
This is a great question that we didn’t explore deeply in the paper. Unlike the developing fly wing disc, where some cells express Notch ligand and others Notch receptor to form a boundary, in our setting all cells are expressing ligand and Notch receptors. Therefore, we believe we are looking at a homeostatic form of Notch signaling contrasting the traditional boundary sender-receiver model in development. To answer your question, all cells within the monolayer in our model likely express the ligand. Which ligand, and how it is being regulated during epithelial restructuring to activate Notch1 cortical signaling are important open questions.
Finally, it seems like your paradigm is primarily modeling development. Do you think Notch cortical signaling is important to maintenance of adherens junctions once they are established?
While our organotypic mammary duct model was central to identifying the morphogenic consequences of loss of Notch1 cortical signaling (compared to traditional in vitro culture), this is still a far cry from development. In actuality, what we identify in our recent paper seems to be more of a homeostatic context, where cortical signaling is actively maintaining epithelial organization and tissue architecture (and transcription is not active!). In this context, Notch cortical signaling is central to maintain adherens junctions once they are established. However, as I mentioned above, we are now applying in vivo and in vitro models of epidermal development to parse the relative contributions of Notch/FAM83H on cell fate and cell adhesion during development.
Figure: How cortical Notch1 regulates cell shape and adherens junctions.